KClO3 Molar Mass: Quick Calculation Guide Understanding KClO3 Molar Mass in Chemistry How to Find KClO3 Molar Mass Easily KClO3 Molar Mass Explained Simply Essential Guide to KClO3 Molar Mass Calculation
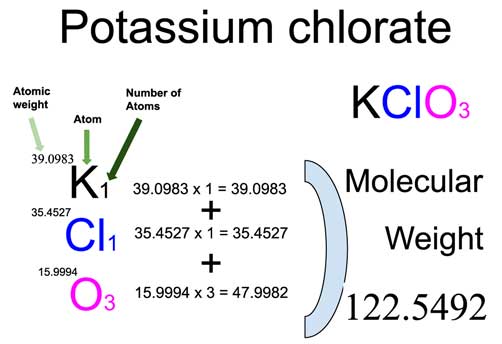
Understanding the molar mass of compounds is crucial in chemistry, as it helps in various calculations and experiments. KClO3, or potassium chlorate, is a common chemical compound used in laboratories and industries. In this guide, we’ll walk you through a quick and easy way to calculate the KClO3 molar mass, ensuring you grasp the concept effortlessly. Whether you’re a student, researcher, or chemistry enthusiast, this post will simplify the process for you.
Understanding KClO3 Molar Mass in Chemistry
The molar mass of a compound is the sum of the atomic masses of all the elements in its chemical formula. For KClO3, it consists of potassium (K), chlorine (Cl), and oxygen (O). Knowing the molar mass is essential for stoichiometry, dilution calculations, and more.
How to Find KClO3 Molar Mass Easily
Identify the Atomic Masses:
- Potassium (K): 39.10 g/mol
- Chlorine (Cl): 35.45 g/mol
- Oxygen (O): 16.00 g/mol (there are 3 oxygen atoms in KClO3)
- Potassium (K): 39.10 g/mol
Calculate the Total Molar Mass:
- K: 39.10 g/mol
- Cl: 35.45 g/mol
- O (3 atoms): 3 × 16.00 = 48.00 g/mol
- Total Molar Mass = 39.10 + 35.45 + 48.00 = 122.55 g/mol
- K: 39.10 g/mol
📌 Note: Always ensure you use accurate atomic masses from the periodic table for precise calculations.
KClO3 Molar Mass Explained Simply

The molar mass of KClO3 is 122.55 g/mol. This value represents the mass of one mole of potassium chlorate. It’s a fundamental unit in chemistry, allowing you to convert between mass, moles, and particles in reactions.
Essential Guide to KClO3 Molar Mass Calculation
To master KClO3 molar mass calculation, follow these steps:
- Step 1: Write down the chemical formula (KClO3).
- Step 2: Look up the atomic masses of K, Cl, and O.
- Step 3: Multiply the atomic mass of each element by its subscript in the formula.
- Step 4: Add the results to get the total molar mass.
| Element | Atomic Mass (g/mol) | Subscript | Contribution to Molar Mass (g/mol) |
|---|---|---|---|
| K | 39.10 | 1 | 39.10 |
| Cl | 35.45 | 1 | 35.45 |
| O | 16.00 | 3 | 48.00 |
| Total Molar Mass | 122.55 | ||

Key Takeaways:
- The molar mass of KClO3 is 122.55 g/mol.
- Accurate atomic masses are essential for correct calculations.
- This knowledge is vital for stoichiometry and lab work.
For those looking to purchase KClO3 for experiments, ensure you source it from reliable suppliers to maintain purity and safety.
What is the molar mass of KClO3?
+The molar mass of KClO3 is 122.55 g/mol.
Why is KClO3 molar mass important?
+It’s crucial for stoichiometry, dilution calculations, and understanding chemical reactions.
How do I calculate the molar mass of any compound?
+Sum the atomic masses of all elements in the compound, considering their subscripts.
In summary, calculating the KClO3 molar mass is straightforward once you understand the basics. By following the steps outlined, you’ll be able to determine the molar mass of any compound with ease. This knowledge is invaluable in chemistry, whether for academic studies or practical applications.
Related Keywords: KClO3 molar mass calculation, potassium chlorate formula, atomic masses in chemistry, stoichiometry basics.



